AA: Nitric acid and nitrate ions
|
Nitrate(V) ion, NO32− (Nitrate ion) |
||
|
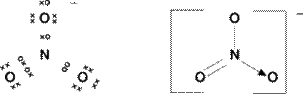
The first
diagram is a dot-cross diagram or a Lewis Structure as they are sometimes
called. It shows the individual
electrons involved in bonding. The
second image shows more clearly whether bonds are single, double or dative
covalent. |
|
|
|
These
images are called resonance structures.
They show that two of the electrons in the double bond are delocalised
over the whole ion. |
|
|
|
The delocalised electrons
are shown as dashed lines in these structures. The first image shows the charge on the ion
is −1 and the second shows the bond angles in the planar ion. |
|
|
|
Nitrate(III) ion, NO2− (Nitrite
ion) |
|
|
|
Nitrogen dioxide, NO2 (Nitrogen[IV]
oxide) |
||
|
NO2
is actually a radical (having an unfilled octet). Therefore, it doesn't
really have a proper Lewis structure. Because of the unfilled octet, NO2
has a tendency to dimerize to N2O4 in an attempt to
attain a fully filled octet around each nitrogen atom. Oxidation state of N: +4 |
|
|
The reaction of copper
metal with nitric acid:
Cu + 2 HNO3 + 2 H+ → Cu2+
+ 2 NO2 + 2 H2O
Some of the nitrate ions form copper(II)
nitrate, Cu(NO3)2.
|
|
Cu(s) |
+ |
4 H+(aq) |
+ |
2 NO3-(aq) |
→ |
Cu2+(aq) |
+ |
2 NO2(g) |
+ |
+ 2 H2O(l) |
|
Ox. state |
(0) |
|
(+1) |
|
(+5) |
|
(+2) |
|
(+4) |
|
(+1) |
Cu2+(aq) + 4 NH3(aq)
→ Cu(NH3)42+(aq)
Concentrated nitric acid is a very strong oxidising
agent.
It is capable of reacting with the noble metals such
as copper or silver.